
JAK Inhibitors in the BSRBR-RA
Read about some of the recent research from BSRBR-RA looking at JAK inhibitors.
Bit of background about you
Hello, my name is Zixing Tian, I am an epidemiologist with a research focus on rheumatoid arthritis (RA). I work on the BSRBR-RA study, where I recently published an analysis on how patients with RA using Janus kinase (JAK) inhibitors are affected by the European Medicines Agency (EMA) advisory change.
where I recently published an analysis on how patients with RA using Janus kinase (JAK) inhibitors are affected by the European Medicines Agency (EMA) advisory change.
What was known about the topic already
JAK inhibitors are the newest type (or class) of advanced RA treatment and have been available since 2017. Four JAK inhibitors have been approved by the EMA which evaluates and supervises medicine use in the European Union. These include Jyseleca, Olumiant, Xeljanz and Rinvoq.
In 2023, the EMA limited the use of JAK inhibitors to certain high-risk patients unless no other suitable treatments are available, due to evidence suggesting that JAK inhibitors increase the risk of cardiovascular disease or cancer compared to TNF inhibitors (another advanced RA treatment). This advisory change was also adopted by the UK Medicines and Healthcare Products Regulatory Agency (MHRA). The four at-risk patient groups include:
- age 65 years or above,
- increased risk of major cardiovascular problems,
- current or past smokers,
- increased risk of cancer.
What question did you want to solve?
I wanted to investigate how many RA patients using JAK inhibitors in the UK do not fit the new safety guidelines from the EMA. This will help us understand how these changes might affect JAK inhibitor prescriptions now and in the future.
What data you looked at and what you found
I used the BSRBR-RA data, which is a national cohort study, established in 2001, of over 30,000 patients with RA on biological therapies (otherwise known as Biologic Disease Modifying Anti-Rheumatic Drugs or bDMARDs) and JAK inhibitors. This cohort study has been recruiting RA patients using JAK inhibitors since 2017. The four other classes of targeted therapies (bDMARDs) have been available for much longer than the JAK inhibitors and the BSRBR-RA has captured extensive long-term data showing the safety and effectiveness of the bDMARDs.
We found that among 1,341 RA patients who had started the JAK inhibitors before EMA advisory change, 80% (1,075 patients) met at least one EMA risk criterion. Of these high-risk patients:
- 49% (529 patients) used JAKi as their first or second treatment type. Suitable alternatives might likely have existed for them, and re-evaluation of the suitability of their treatment may be needed.
- 28% (299 patients) had already tried at least three out of four other advanced RA treatment types. For these patients, options for alternative therapies would be very limited.
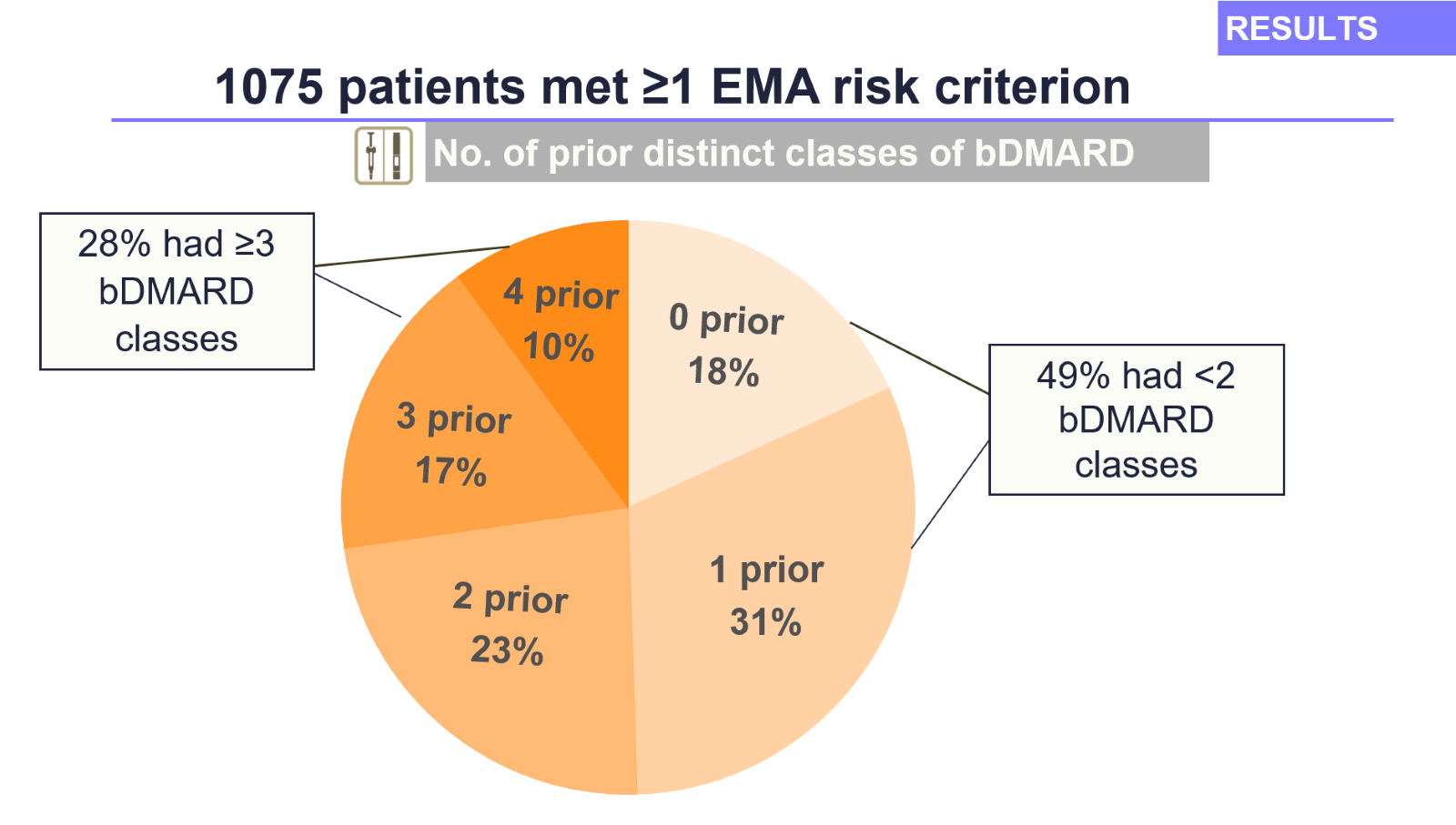
Figure Number of prior distinct of bDMARDs in patients who had ≥1 EMA risk criterion (N=1075)
Looking to the future – any further work on this
This is just the start of the JAK inhibitor story in the BSRBR-RA data and I would like to go on to look at the following once more data has been collected:
- Analysis of treatment data collected after January 2023 will help understand the impact of licensing recommendations and shifts in the practical use of JAK inhibitors.
- Comparing the risk of cardiovascular disease and cancer between JAK inhibitors and the other four types of advanced RA treatments.






0 Comments