Participant Data Journey -what happens to the data?
Have you ever wondered what happens to the data that is collected as part of the BSRBR-RA Study? We have recently published a new data journey to help our participants to understand the impact that their data has had on the real world of rheumatology.
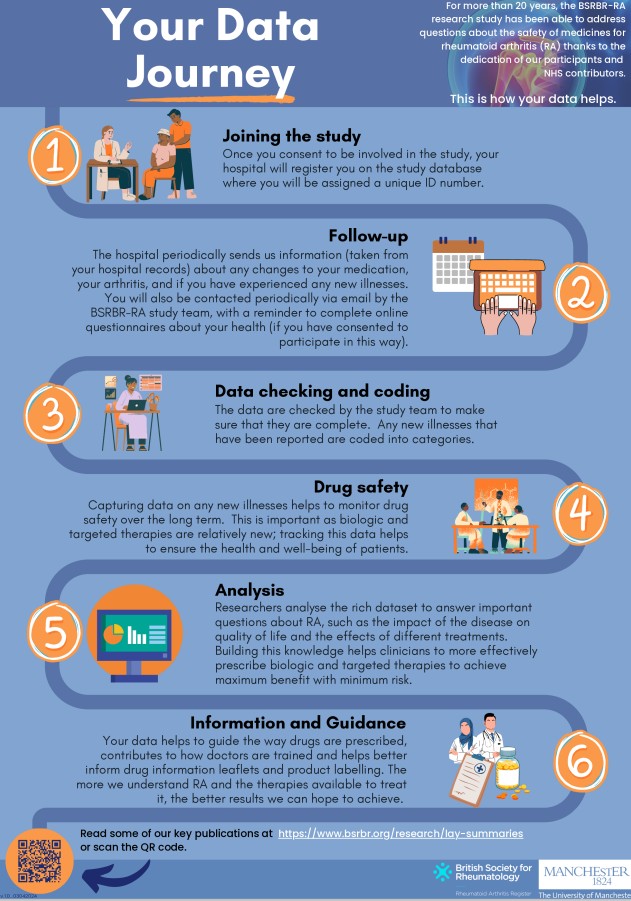
Joining the study
If your hospital is set up to recruit to the BSRBR-RA, it is very easy to participate in the study. When you start a new biologic, biosimilar or other targeted therapy for your rheumatoid arthritis, you may be eligible to be enrolled.
Once you have read the Patient Information Sheet and signed the consent form, your hospital will register you in the study.
Follow up
Periodically, your hospital will send BSRBR-RA information from your hospital records. This information is to do with:
- Any changes to your medication for rheumatoid arthritis.
- Whether you have had any new illness or medical conditions.
- How your arthritis is generally.
If you have consented to direct patient follow up, you will receive an email to complete online questionnaires about your health.
What happens to the data?
The study team in Manchester check all the incoming data for quality and accuracy.
 Then, the data is grouped together in a ‘dataset’. This is anonymised and no one can be identified. Researchers analyse the data to answer important questions about rheumatoid arthritis – the impact it has on your health and quality of life and the effects of different treatments.
Then, the data is grouped together in a ‘dataset’. This is anonymised and no one can be identified. Researchers analyse the data to answer important questions about rheumatoid arthritis – the impact it has on your health and quality of life and the effects of different treatments.
What has been discovered so far?
So far, analyses using BSRBR-RA study data has contributed to: –
- The way drugs are prescribed.
- How doctors are trained.
- Drug information leaflets.
There have been over 100 publications using BSRBR-RA data. Read some of our lay summaries to find out more key findings.
More information for participants of BSRBR-RA can be found on our website.






0 Comments